|
NMRPredict:
|
|
Stereochemistry - Triterpene example
Look at the triterpene molecule below. Atoms 30 and 31 should be predicted very differently because of stereochemistry. However, if stereochemistry
is not taken into account (as in this screen shot) both atoms are predicted at 27.4 ppm.
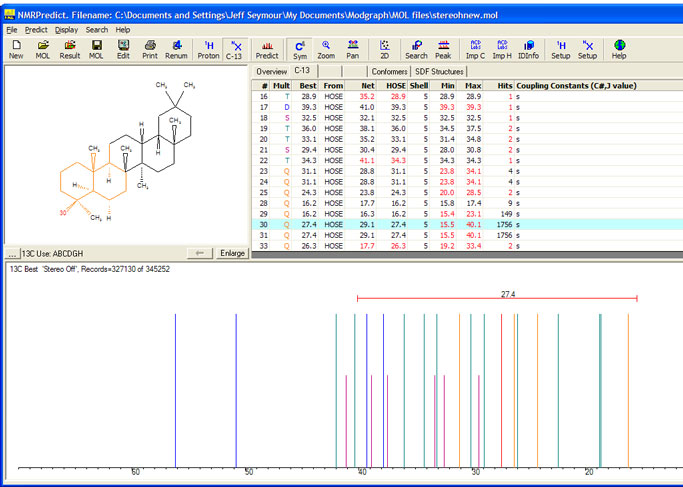
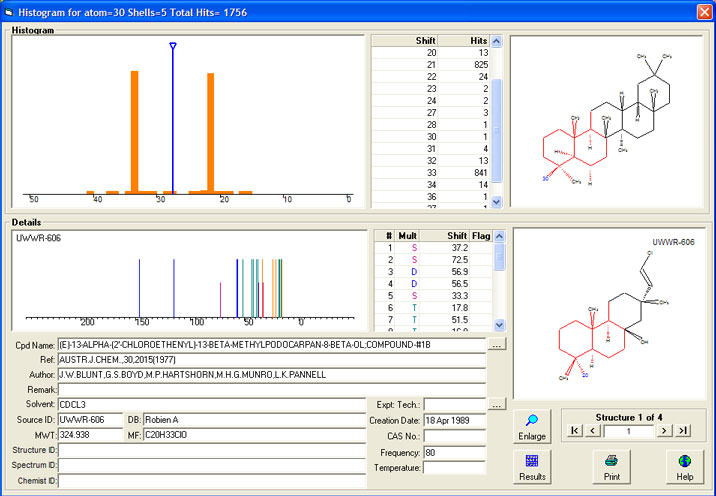
Looking at the histogram display of the database records used shows that without stereochemistry a "mean value" is often a "meaningless value".
95% of the database records either fall at 21 ppm or 33 ppm depending on stereochemistry. The HOSE code without stereochemistry will, in principle,
simply add 21 to 33 and divide by two, giving a predited value of 27 ppm.
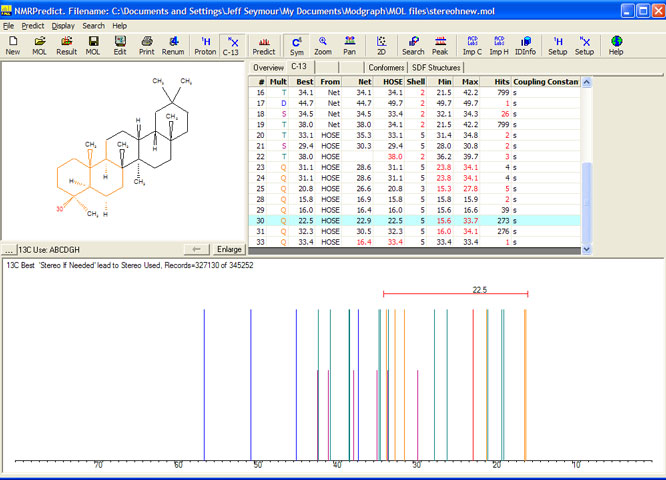
As soon as stereochemistry is turned on in NMRPredict (see the result below) atoms 30 and 31 are predicted more than 10 ppm apart. NMRPredict is intelligent enough to evaluate each query molecule and decide whether stereochemistry
should be used during prediction or not.
|



