| Home | Products | Consulting Services | Contact us |
NMRPredict:
| Modgraph Home | ||
| NMRPredict Overview | ||
| Carbon 13 NMR Prediction Overview |
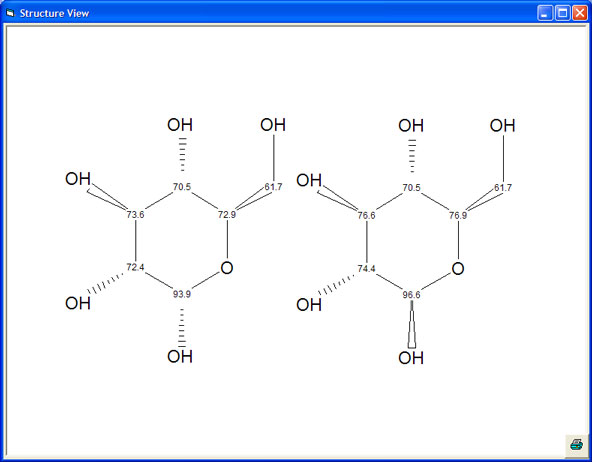
Stereochemistry - Sugar
The example below shows how critical it is to use stereochemistry in the prediction of sugars.
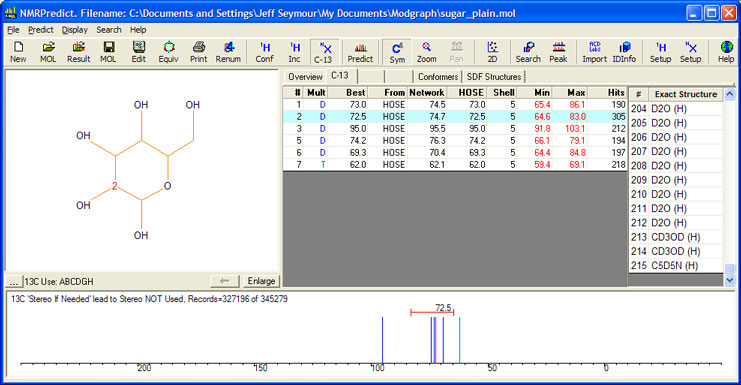
The prediction of the flat molecule, with no stereochemistry drawn, shows that there are 215 exact structures in the database which match the sugar and that the range of values used for prediction varies from 10 to 21 ppm. There can be no confidence in a prediction with such a range and the prediction is of no value for assignment and structure verification.
By (a) drawing the molecule with stereochemistry and (b) selecting only records from the database measured with water as the solvent - only 7,428 of the potential 345,279 records - gives a prediction range of only 3 ppm. There can be a good deal of confidence in such a prediction.
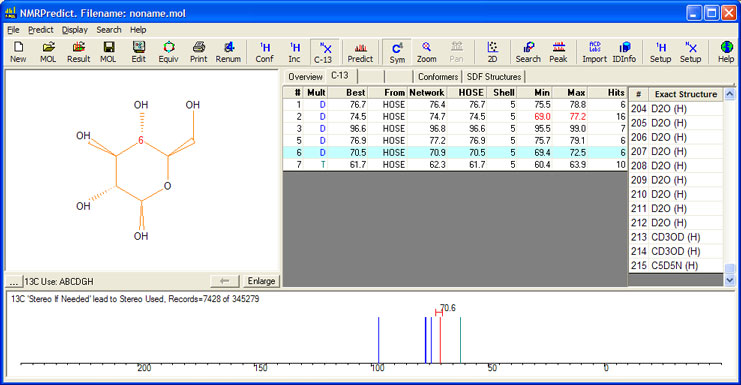
It can also be seen that NMRPredict is able to differentiate between the alpha and beta configurations of the molecule.