| Home | Products | Consulting Services | Contact us |
NMRPredict:
| Modgraph Home | ||
| NMRPredict Overview | ||
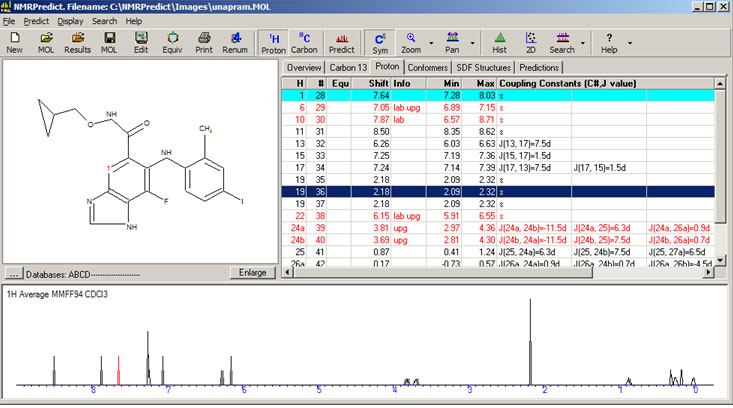
| Proton NMR Prediction Overview |
Parametrised functional groups
Professor Abraham has been parametrising functional groups in CHARGE for over 20 years. The process for parametrising is:
- Each functional group is identified and treated separately
- A range of compounds with fixed geometry is selected
- 3-D structures are retrieved using ab initio or MM calculations
- The 1H NMR data is retrieved and assigned
- The relevant parameters are varied to reproduce the experimental data
Parametrised groups
The following groups have been parametrised and should give accurate predictions.- Alkanes
- Ethers
- Alkenes
- Aldehydes
- Alkynes
- Ketones
- Aromatics
- Amides
- Halocompounds
- Esters
- Nitriles
- Sulphides
- Nitro compounds
- Alcohols
- Amines
- Heteroaromatics(inc 5 and 6 membered rings)
- Unsaturated ketones
- Halo-olifins
- Sulphoxides
- Sulphones
Non parametrised groups
The following groups have not yet been parametrised and predictions cannot be relied upon at all. NMRPredict will give a warning when any such groups are contained in a query molecule by marking the predicted atom in red.- 4 membered heterocyclic rings
- Azo compounds
- Silicon compounds
- Phosphorus compounds
- Charged compounds (salts, amino acids)